2023 PBS changes
This page summarises changes to how opioid dependence treatments will be provided from July 1, 2023, and provides brief guides to pharmacists and prescribers.
Note that doctors, nurse practitioners and pharmacists must continue to comply with all relevant State legislation regarding the prescribing, dispensing, recording and storage of drugs of dependence. The Victorian State Government DoH have published a fact sheet, Features of a Victorian Pharmacotherapy Prescription to assist with this.
For complete guides and rules about the changes, refer to the PBS and Pharmacy Programs websites.
On July 1, 2023, new PBS and Pharmacy Programs arrangements came into effect, changing the way pharmacies, doctors and nurse practitioners provide treatments for opioid dependence.
The new arrangements provide for listing of oral liquid methadone, sublingual buprenorphine and buprenorphine/naloxone, and long-acting injectable buprenorphine under the Highly Specialised Drugs, Community Access section of the PBS schedule.
From July 1, 2023:
- Patients with a Medicare card are eligible to pay a single PBS co-payment for 28 days supply of the listed treatments. Multiple co-payments are payable if multiple strengths are supplied.
No further fees are payable by the patient to access the treatment. - For new prescriptions after July 1, doctors and nurse practitioners must provide their patients with a valid PBS prescription for up to 28 days supply, plus 2 repeats, if required.
- Pharmacists will be remunerated for PBS supplies under the usual arrangements
- A daily fee per patient, for activities associated with the staged supply of these medications (excl. LAIB) and an administration fee for LAIB, if applicable, will be claimable by participating pharmacies via the Pharmacy Programs portal,
Fact sheets
The Federal government and pharmacy programs administrators have published fact sheets relating to the new PBS and Pharmacy Programs arrangements. Links to the fact sheets are provided below, or may be accessed via the PBS dedicated web page here – https://www.pbs.gov.au/browse/section100-md
- ODT Overview Factsheet (PDF 209KB)
- Information for prescribers (PDF 322KB)
- Information for community and hospital pharmacists (PDF 209KB)
- ODT dispensing workflow and stock management for pharmacists (PDF 330KB)
- Information for patients in methadone or buprenorphine programs (PDF 151KB)
- ODT medicine transition arrangements for private clinic and non-PBS approved dosing sites (PDF 186KB)
- ODT Frequently Asked Questions (PDF 203KB)
Find guides and rules associated with the Opioid Dependence Treatment Pharmacy Program at the PPA website here – https://www.ppaonline.com.au/programs/medication-adherence-programs-2/odt
Information related to transition arrangements is now redundant, and has been removed from this guide, as all pre-July 2023 prescriptions will be either fully supplied, or are no longer valid.
New prescriptions written after July 1
New prescriptions for the PBS-listed opioid pharmacotherapy items should be PBS streamline authority prescriptions, with exact quantities for up to 28 days supply and two repeats OR for patients on methadone doses greater than 150mg, or sublingual buprenorphine doses greater than 32mg, a PBS authority prescription with telephone approval for an increased quantity.
Authorities for increased quantities of the long-acting buprenorphine injections are not provided for, but note that the usual rules for early supply are allowed, enabling more frequent injection for those patients that require it (typically between every 21 to 28 days).
Note that all state requirements and guidelines for the writing and dispensing of drugs of dependence prescriptions continue to apply or are recommended,
Writing valid PBS prescriptions for ODT.
After July 1, 2023 you must provide PBS prescriptions for ODT treatments. Pharmacists will be unable to dispense prescriptions as PBS if not correctly written.
Refer to tables below for maximum quantities and streamline authority codes.
- Calculate the quantity of medication required for up to 28 days supply.
- For patients requiring less than or equal to the maximum quantity for up to 28 days’ supply, provide a streamline authority paper or e-script for the calculated quantity plus up to 2 repeats,
OR
For patients requiring greater than the maximum quantity for up to 28 days’ supply (patients taking more than 150mg methadone or 32mg SL buprenorphine daily), obtain a telephone authority for the increased calculated quantity plus up to 2 repeats.
- In the case of sublingual buprenorphine preparations, where more than one strength of buprenorphine is required, you must provide a prescription for each strength to be supplied.
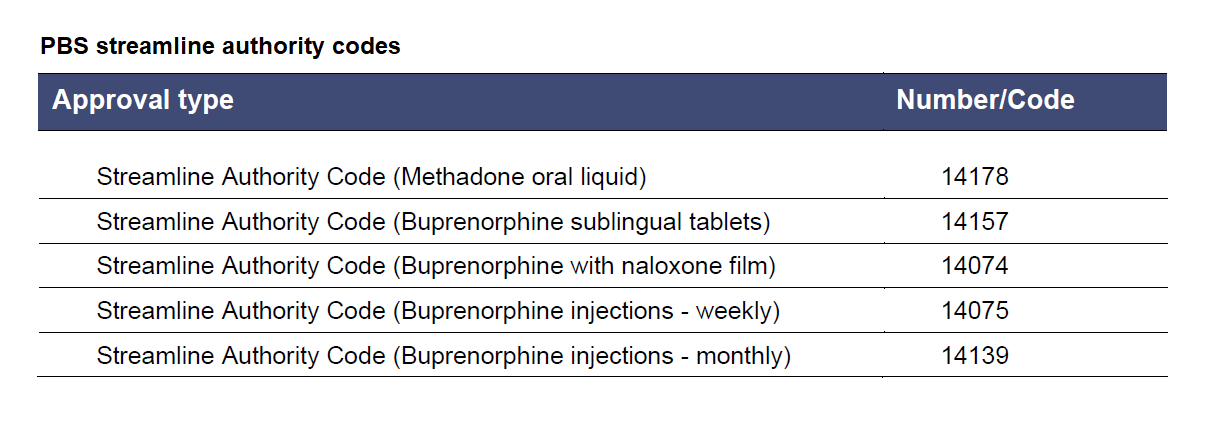
Table from PBS Fact Sheet for Prescribers

Prescribing ‘monthly’ long-acting buprenorphine (LAIB) where the dose interval is less than 28 days
It should be noted that increased quantity authorities are not provided for LAIB, but this does not prevent prescribing a reduced dose interval for the ‘monthly’ injection (less than 28 days) when clinically appropriate. Prescribe a single injection and repeats and indicate the reduced interval clearly on the prescription. Your pharmacist is able to supply at the reduced interval under normal PBS rules.
Your patient having more frequent injections will also require more frequent new prescriptions, due to the reduced interval. e.g a patient having an injection supplied and administered every 21 days will need a new prescription at around every 63 days, presuming 1 injection and 2 repeats are prescribed.
Direct supplies of pharmacotherapy agents to doctors and nurse practitioners
Some doctors and nurse practitioners may obtain direct supply of pharmacotherapy agents, primarily LAIB. Transition arrangements allow for continued direct supply.
Clinicians currently receiving direct supply are encouraged to arrange alternative supply from a PBS accredited provider (a community pharmacy or approved hospital pharmacy) before the end of the transition period.
Quick links
Tips for writing valid PBS
opioid pharmacotherapy prescriptions NEW!
Websites
PBS opioid dependence treatments website
Pharmacy Programs Opioid Dependence Treatments
Victorian State Government DoH Pharmacotherapy Policy – updated for new PBS rules
Fact sheets
Victorian Government fact sheets
Features of a Victorian Pharmacotherapy Prescription – a guide for prescribers to comply with Victorian prescription requirements.
PBS Fact sheets
These are direct download links from the PBS website.
ODT Overview Factsheet (PDF 209KB)
Information for prescribers (PDF 322KB)
Information for community and hospital pharmacists (PDF 209KB
Information for patients in methadone or buprenorphine programs (PDF 151KB)
ODT dispensing workflow and stock management for pharmacists (PDF 330KB)
ODT medicine transition arrangements for private clinic and non-PBS approved dosing sites (PDF 186KB)
ODT Frequently Asked Questions (PDF 203KB)
Key points for prescribers
- From July 1, 2023 new prescriptions must be PBS.
- Up to 28 days supply, plus 2 repeats are allowed.
- Prescribe precise quantities.
- For sublingual buprenorphine, provide a prescription for each strength required.
- All new PBS items are streamlined authority.
- Doses greater than 150mg for methadone or 32mg for buprenorphine will require telephone authorities for increased quantity.
- Increased quantity authorities are not allowed for LAIB, but early supply by pharmacists is allowed, enabling more frequent dosing when clinically appropriate
Key points for pharmacists
- A co-payment is payable by the patient for each 28 day supply, and for each strength supplied (for SL buprenorphine preparations).
- A daily fee for staged supply activities is claimable in arrears via the PPA portal.
- No further fees are payable by the patient.
- Early supply is allowed under the same rules as other PBS medicines.